Safeguarding Health & Security
Avicena empowers nations and organisations to stay ahead of emerging threats with agile, high-performance outbreak preparedness — enabling rapid detection and containment.

Preparedness Solution
a. Dewhurst, R.E., Heinrich, T., Watt, P. et al. Validation of a rapid, saliva-based, and ultra-sensitive SARS-CoV-2 screening system for pandemic-scale infection surveillance. Sci Rep 12, 5936 (2022). https://doi.org/10.1038/s41598-022-08263-4.
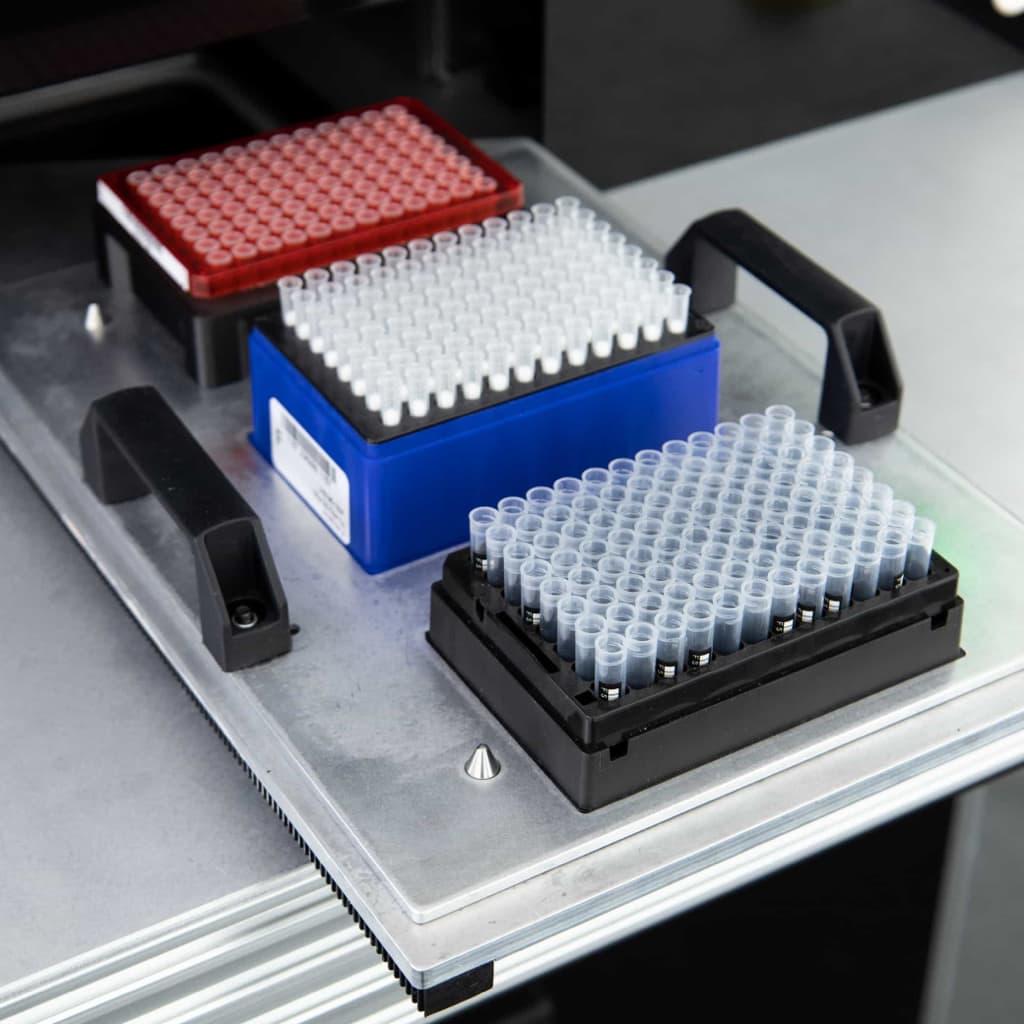
Preparedness Solution
A continuous reagent supply system designed to keep nations and organisations ready for known and emerging pathogenic threats.
The Avicena Smart Stockpile System minimises waste from expired reagents and ensures a steady, uninterrupted supply of test materials, so your biosecurity readiness is always assured.
The system supports rapid activation of targeted test kits when new threats emerge, enabling seamless scaling of diagnostic capacity.

Avicena’s outbreak preparedness solutions enable you to connect human, animal, and environmental health. Sentinel’s high-throughput diagnostics and Smart Stockpile’s continuous supply readiness combine to form a scalable, adaptable foundation for national biosecurity programs.
This integrated strategy enables rapid detection, reporting, and response across sectors, ensuring continuity of critical operations while protecting public health and economic stability.
Identify and contain pathogens in minutes, not days, ensuring swift action to protect communities and economies.
Supports a broad range of infectious agents, including avian flu, MERS, SARS-CoV-2, Influenza A & B, and more.
From airports and food processing plants to animal health and environmental monitoring, Sentinel solutions scale to meet the needs of any setting.
Reduce waste and costs with on-demand testing, Smart Stockpile solutions, and cloud-integrated workflows.
Be prepared to detect, respond to, and contain outbreaks—before they escalate.
Contact our team to discuss how Avicena’s integrated solutions can advance your national biosecurity strategy.

Avicena’s Biosecurity Solution is designed to support large-scale pathogen screening with a focus on speed, scalability, and operational readiness.
The Sentinel system enables high-throughput molecular screening with PCR-comparable accuracy, delivering results efficiently to support timely public health decisions. Our Smart Stockpiling System supports proactive preparedness by ensuring reagents and consumables are available ahead of time.
The integrated solution provides not only diagnostic instruments, but a standby testing capability and logistical framework that can be deployed rapidly in response to emerging pathogen threats.
If you are already equipped with Avicena’s Smart Stockpiling System, your testing capability can be activated immediately, on day zero.
For new customers, Avicena can manufacture and deliver a Sentinel Mini within three weeks, and a Sentinel ULTRA within four weeks, along with an agreed volume of test assays and consumables.
Each unit offers high-throughput capacity of up to 5,000 tests per hour with Sentinel ULTRA, or 384 tests per hour with Sentinel Mini. When combined with the Smart Stockpiling System, this enables immediate surge testing with no reliance on external supply chains or additional staffing.
Traditional supply chains are reactive, they fail when global demand spikes.
Avicena’s Smart Stockpiling System utilises non-perishable microplates, shelf-stable base reagents, and rapid custom primer delivery to keep your system on standby and minimise waste. Expired stock is automatically replaced on a rolling basis, ensuring continuous readiness.
Avicena supports a wide range of pathogens across respiratory, vector-borne, and bioterrorism-related diseases, including:
Our platform is pathogen-agnostic, meaning new assays can be deployed rapidly in response to emerging threats.
Sentinel is available in both lab-grade and rugged, field-ready formats. Whether deployed in a central lab, border checkpoint, defence base, or regional clinic, both the Sentinel ULTRA and Sentinel Mini offer a portable, easy-to-use solution suitable for remote operations.
The best fit depends on your required testing volume, speed, and level of automation.
ISO 13485 certified for the design, development, and manufacture of in vitro diagnostics
CE-Marked for use in the in vitro diagnostic (IVD) molecular testing
Avicena holds several patent pending innovations that enable our groundbreaking performance.
Validation of a rapid, saliva-based, and ultra-sensitive SARS-CoV-2 screening system for pandemic-scale infection surveillance. Dewhurst RE, et al. 2022. Nature, Scientific Reports.
The Avicena Sentinel instrument is intended for trained laboratory professionals to run human IVD isothermal amplification assays. Diagnostic use of the Sentinel requires IVD reagents and laboratory workflows which have been authorised by relevant regulatory agencies in each jurisdiction and by Avicena Systems as compatible with the instrument.
The Avicena Sentinel instruments are also available in configurations for research use, environmental/surveillance, animal health screening, and food safety testing.
AVICENA SENTINEL IS NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.
Avicena Sentinel is for in vitro diagnostic use in humans and animals.
Avicena Seguro is for animal health and research use only (RUO).
Contact an Avicena Systems representative for regional availability.